Reactive Inorganic Chemistry
In collaboration with the Cummins group (MIT), we investigate several reactive inorganic compounds. Microwave spectroscopy is an invaluable tool to complement standard mass spectroscopic or NMR measurements, as it allows a non-ambiguous identification of the molecules probed (nature, connectivity, structure) as long as they possess a permanent dipole moment and that their rotational transitions fall in the microwave region.
RSNOs formation and reactivity
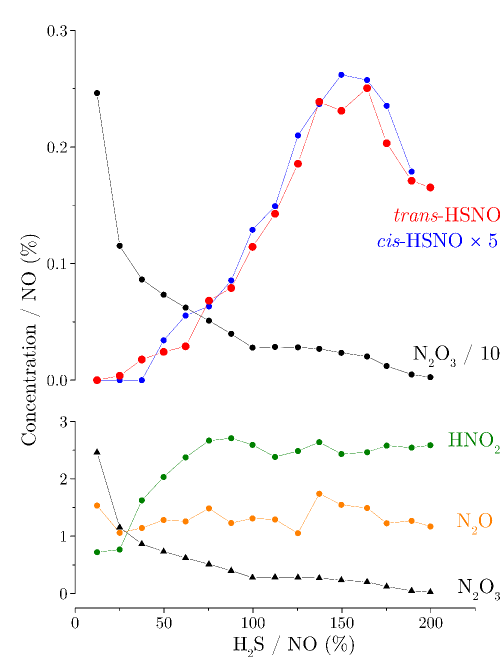
The vast majority of NO in vivo is bound as S-nitrosothiols (RSNO), a family of molecules well known for their crucial role in response to oxygen deprivation as well as for being important biological reservoir for NO. RSNOs are typically formed through the reaction of nitrosylating agents with thiols (RSH).
We study the formation of RSNOs from RSH and NO, and their subsequent reactivity by means of Fourier-transform microwave spectroscopy. Our results show that for the two simplest RSH molecules, H2S or CH3SH, when a mixture of RSH and NO heavily diluted in an inert buffer gas is expanded into a vacuum chamber, intense rotational lines of RSNO can be detected.
Our measurements reveal that HSNO is formed with high efficiency by the surface-catalyzed reaction RSH + N2O3 → RSNO + HNO2, where N2O3 is a product of NO disproportionation. Our studies also suggest that, in case of H2S, further reaction of HSNO with H2S may form HNO and HSSH.
Reactive intermediates from molecular precursors
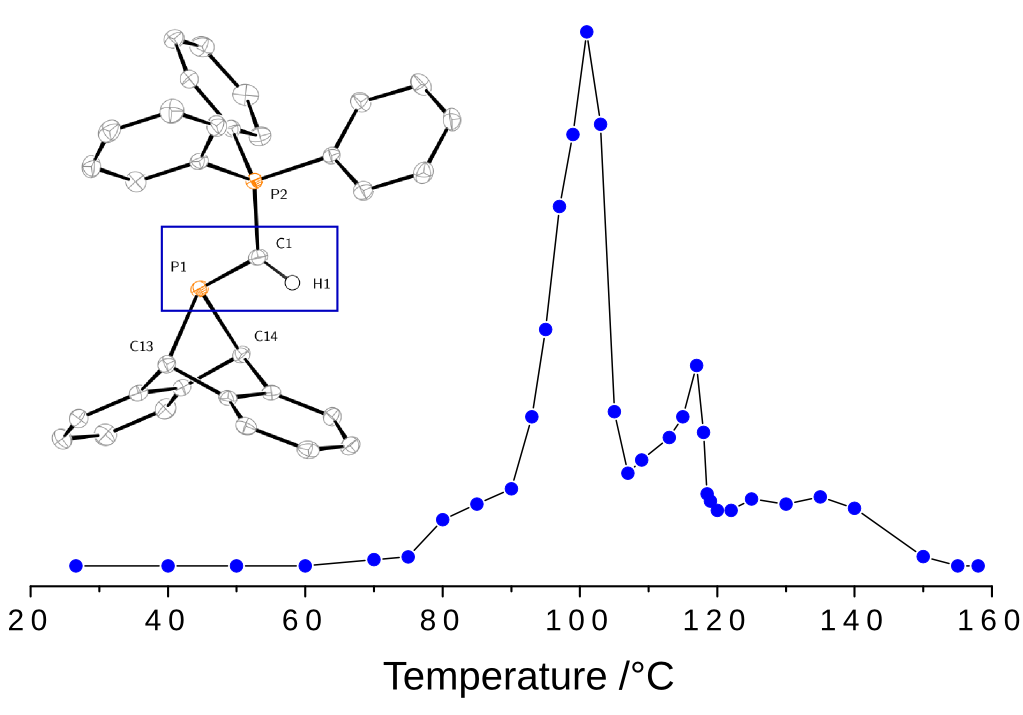
The Cummins group (MIT) is engaged in the design and synthesis of novel molecular precursors providing access to species which themselves are too unstable to be stored in a bottle. When heated under mild conditions, the molecular precursors release reactive small molecules which have importance in synthetic chemistry and may have potential astrophysical interest. These released species can then be proved using microwave spectroscopy.
The method is an alternative to conventional production of unstable species using electrical discharge in stable precursors which, compared to that latter method, presents the advantage of being highly specific: the species released by heating are known in advance.
Find more about this project:
A molecular precursor to phosphaethyne and its application in synthesis of the aromatic 1,2,3,4-phosphatriazolate anion. W. J. Transue, A. Velian, M. Nava, M. A. Martin-Drumel, C. C. Womack, J. Jiang, G.-L. Hou, X.-B. Wang, M. C. McCarthy, R. W. Field, and C. C. Cummins. Journal of the American Chemical Society 138, 6731 (2016)

